For innovative drug development, we can provide study designs, sample size estimations, protocols, and analysis plans for any kind of clinical pharmacology study, including:
For generic or supergeneric drug development, we can define your bioequivalence study program, including Therapeutic Equivalence studies, using a pharmacodynamic endpoint or marker if needed.
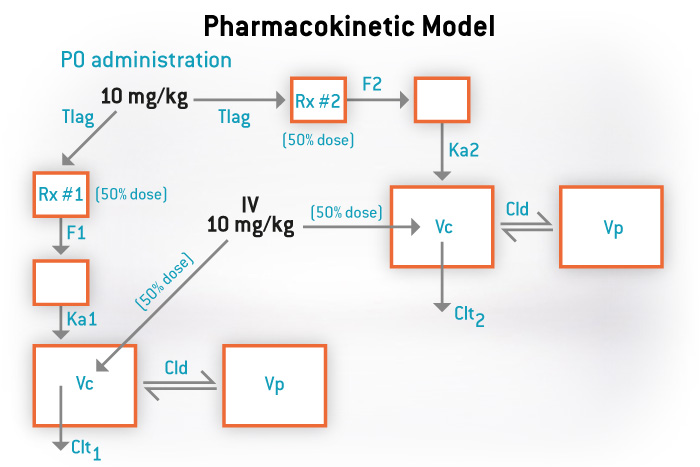
We also offer a variety of services to meet all your pharmacokinetic needs
We provide pharmaceutical project management and other in-house support services that range in scope, such as:
Predictions of drug concentration-time profiles and dosing regimens for various levels of renal and/or hepatic function.